Real4Reg is working to improve how real-world health data (RWD) is used in decisions about medicines and medical technologies across Europe. The project focuses on developing and applying artificial intelligence (AI) to make data analysis more effective and reliable throughout the entire product lifecycle. The results will help shape training and good practice guidelines for both regulators and health technology assessment (HTA) bodies—ultimately supporting better decisions and improving patient care.
To do this, Real4Reg brings together data from a variety of national sources to build a strong, trustworthy RWD foundation. Researchers are using health insurance data in Germany and data from national health registers in Denmark, Finland, and Portugal. All data is handled in line with legal and privacy rules. Each dataset remains within its country of origin and is managed by the organisation that owns it—ensuring secure, decentralised control and accountability.
Shared Data, Shared Goals
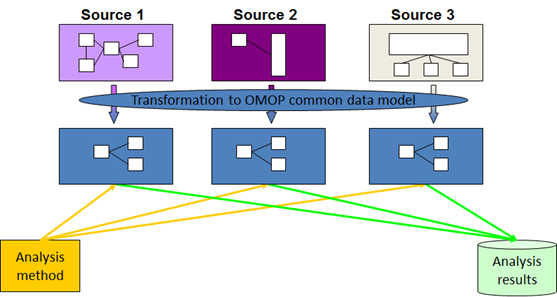
One of the first major milestones in the project was to develop a common data model that could be used across all countries. Since each country collects and structures health data differently, this model helps organise everything in a consistent way. Having a shared structure makes it easier to work with the data—whether that means generating simple summaries, making international comparisons, or applying more advanced AI tools. It also supports transparency and reproducibility, both of which are key to trustworthy, cross-border research.
WP1: Focusing on Breast Cancer and ALS
There are four main use cases in the Real4Reg analysis phase. Work Package 1 (WP1) is currently focusing on two of them—both addressing serious diseases with unmet research needs: amyotrophic lateral sclerosis (ALS) and breast cancer. The two diseases have been chosen because they represent examples of a rare (ALS) and a common (breast cancer) condition with diverse patients’ characteristics, treatment and prognosis. Together the two conditions span the spectrum of real-world data applications.
Use case 1 explores how national health data can help us understand the epidemiology and clinical course of ALS and breast cancer. This includes their incidence, prevalence, and characteristics of the affected patients, as reflected in diverse real-world data types, including national registries and insurance claims data from four countries.
Use case 2 builds on the findings of use case 1 to showcase potential uses of machine learning in the treatment preapproval phase. In the ALS population, machine learning will be used to identify in real world data external control arms for preapproval trials of treatments. In the BC population, machine learning and use of synthetic data will be applied to improve understanding of the disease among men, who represent an neglected and not well understood subgroup of breast cancer patients. If successful, such strategies may help shorten the time for making important treatments available for patients. All analyses will be done using a federated approach using the OMOP common data model to facilitate rational use of resources and enable analysis streamlining and accountability.
WP 1 is a collaborative effort of several expert institutions. The work began under the leadership of the Danish Medicines Agency, and in September 2024, leadership was transferred to the Department of Clinical Epidemiology (DCE) at Aarhus University. Both institutions remain intimately involved in the work. DCE’s mission is to improve clinical care through high-quality clinical epidemiological research, education, and the translation of knowledge into practice. With a vision to be a world-leading department in its field, DCE works in global partnership to address both current and future clinical challenges—making it a strong and fitting lead for WP1. For more information about the department, you can read more here. In addition to the coordinating institutions, WP1 benefits from the active collaboration of a broad range of partners — including academia, regulatory bodies, research centres, and patient organisations across Europe. Learn more about the consortium here.
The next steps in Work Package 1 are to prepare a report on scientific results of the use case 1 and continue with the applications of the machine learning methods – these efforts move us forward in our mission. Stay informed for upcoming updates.
